ChemInform Abstract: Base-Catalyzed Ring Openings of Benzocyclobutenones and -ols.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
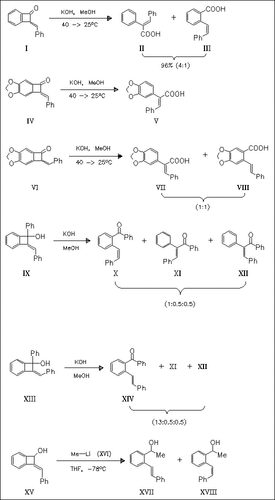
The base-catalyzed ring opening of E- and Z- benzylidenebenzocyclobutenones and -ols is studied in protic and aprotic solvents. Stilbenes are formed by cleavage of the C-1/C-2 bond mainly under retention of stereochemistry. Reaction of (XV) or its E- isomer with MeLi in THF proceeds with isomerization to give the alcohols (XVII) and (XVIII) in both cases.