ChemInform Abstract: Studies on the Stability of δ2 and δ3 Cephem Esters. Part 1. Marked Difference in Stability Between δ2 and δ3 Cephem Prodrug Esters and Application to the Preparation of Key Intermediates for Oral Cephem Synthesis.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
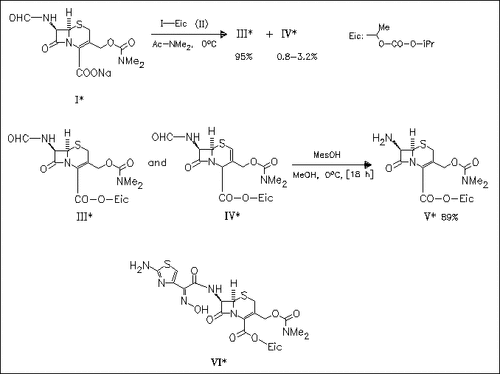
Esterification of the sodium salt (I) affords the δ3 cephem ester (III) together with the inseparable δ2 minor component (IV) . The amount of δ2 ester can be decreased by formamido cleavage at prolonged reaction time leading to the δ3 cephem ester (V), a key intermediate of E1101 (VI), a new oral cephalosporin.