ChemInform Abstract: Reactions of Lignan Precursors, Cinnamyl Alcohols and Cinnamic Acids with Weitz′ Aminium Salt.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
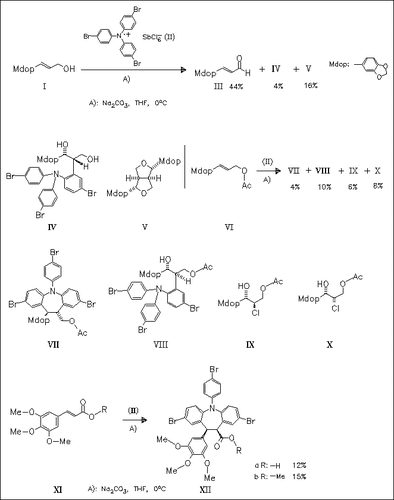
While the reaction of the cinnamyl alcohol (I) with Weitz′ aminium salt (II) affords cinnamaldehyde (III), the coupling product (IV) and ( .+-.)-sesamin (V), the cinnamyl acetate (VI) produces analogous coupling products together with the dibenzoazepine derivative (VII). The cinnamates (XI) react in the same manner to produce the dibenzoazepines (XII) as sole products.