ChemInform Abstract: First Permanent Opened Forms in Spiro(indoline-oxazine) Series: Synthesis and Structural Elucidation.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
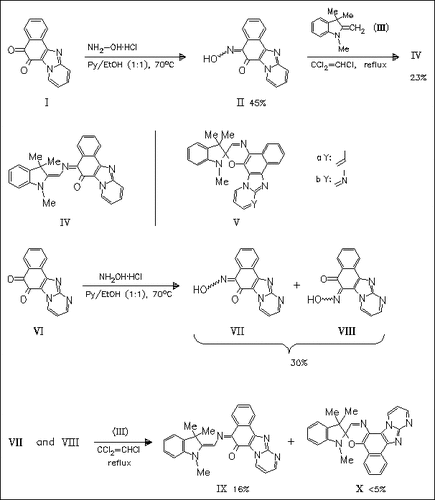
Condensation of oximinonaphthoquinones (II) and (VII) with indoline derivative (III) affords the unexpected, highly colored, first permanent opened forms (IV) (violet) and (IX) (blue) of spirooxazines ( Va) and (Vb). Compound (IX) is accompanied by a small amount of green by-product (X), due to the inseparable mixture of educts (VII) and ( VIII). The geometrical trans-trans-cis structure as well as the quinoidic electronic distribution of (IV) and (IX) are determined by 1H NMR spectroscopy, dipole moment and X-ray diffraction measurements.