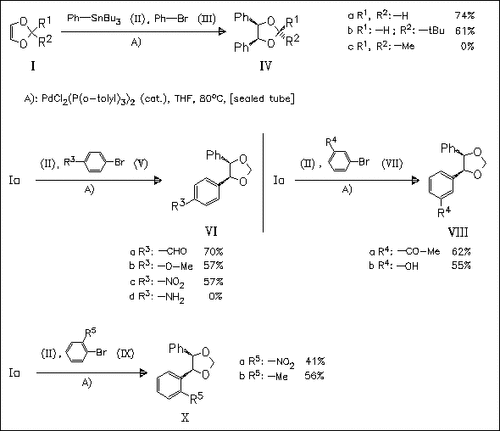
ChemInform Abstract: Palladium-Catalyzed Ternary Coupling Reaction Composed of Aryl Bromides, Phenyltributyltin and 1,3-Dioxole.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The ternary Pd-catalyzed reaction of aryl bromides, dioxoles (I) and phenyltributyltin provides a new synthetic procedure for stereodefined dioxolanes (18 examples).