ChemInform Abstract: Chemoselective Aldol-Type Condensation of Silyl Enol Ethers and Acetals in 5 mol dm-3 Lithium Perchlorate-Diethyl Ether.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
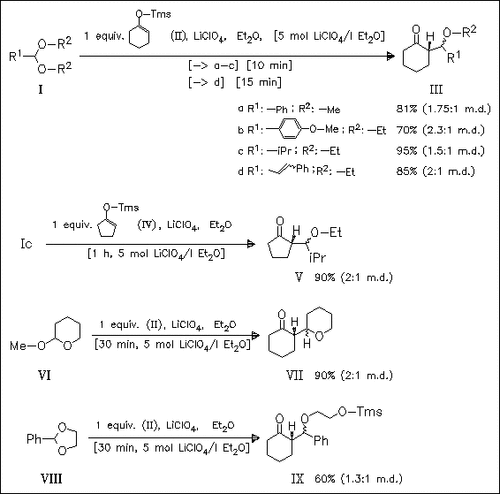
Aldol type condensation of (cyclic) acetals, e.g. (I), (VI) or (VIII) with silyl enol ether (II) under mild Lewis-acidic conditions gives the corresponding aldol ethers with moderate diastereoselectivity. Under the same conditions the parent aldehydes and ketals fail to react. In contrast to (II), which reacts with aromatic and aliphatic acetals equally facile, the corresponding cyclopentenyl ether (IV) does not react with aromatic acetals even after prolonged reaction time.