ChemInform Abstract: Control of the Diastereoselectivity of the Allylation and Deuteration of 2-Hydroxyalkyl Aryl Sulfoxides.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
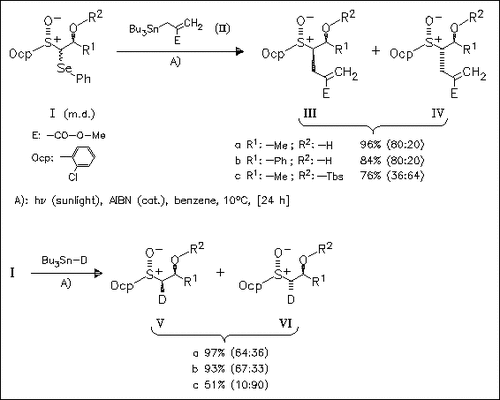
Whereas the radical allylation of the syn educts proceeds with moderate diastereoselectivity, good levels of selectivity can be observed for the anti analogues (Ia) and (Ib). The configuration at C( 1) can be inverted by silyl-protecting of the OH group (→ cf. ( Ic)).