ChemInform Abstract: Enantioselective Syntheses of 2-Arylpropanoic Acid Non-Steroidal Antiinflammatory Drugs and Related Compounds.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
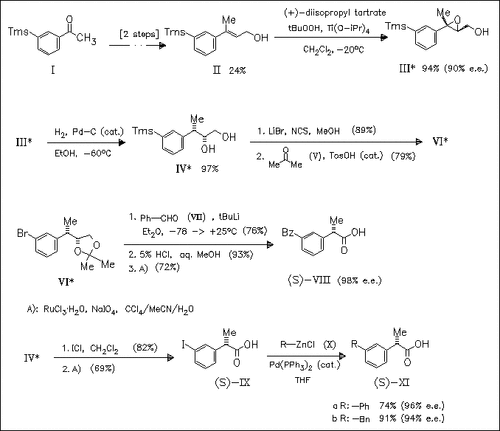
The non-steroidal antiinflammatory drugs ketoprofen (S)-(VIII) and ibuprofen are synthesized in high enantiomeric excess as shown for (S)- (VIII). Control of stereochemistry is achieved by a combination of Sharpless epoxidation followed by catalytic hydrogenolysis of the introduced benzylic epoxide oxygen bond with complete inversion of the stereochemistry. The coupling of organic compounds in the presence of Pd(0) with enantiopure 2-(3- or 4-iodophenyl)propanoic acids, e.g. (S)- (IX), is a general method for the synthesis of optically active arylpropionic acids.