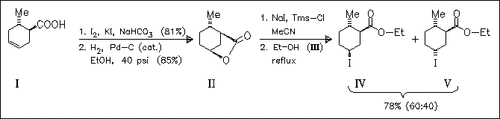
ChemInform Abstract: Regioselective Synthesis of Ceralure B1 and A, Ethyl cis-(and trans-) 5-Iodo-trans-2-methylcyclohexane-1-carboxylate.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
Ring-opening of the lactone (II) by sodium iodide and trimethylsilyl chloride followed by ethanolysis of the resulting silyl ester affords a mixture of the two carboxylates (IV) and (V). Recent studies have shown that only the isomer (IV) is the most active attractant for medflies. The mixture might replace the commercially used attractant trimedlure which contains only 20-25% of active isomers.