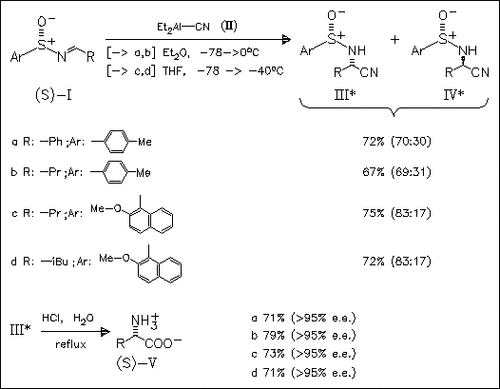
ChemInform Abstract: Asymmetric Strecker Synthesis Using Enantiopure Sulfinimines: A Convenient Synthesis of α-Amino Acids.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The enantiopure sulfimines (I) react stereoselectively with diethylaluminum cyanide (II) to give the diastereomerically enriched . alpha.-amino nitriles (III) and (IV). After chromatographic separation the main isomers (III) are hydrolyzed to the desired α-amino acids.