ChemInform Abstract: Towards Oleananes: Geminal Dimethylation at C-4.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
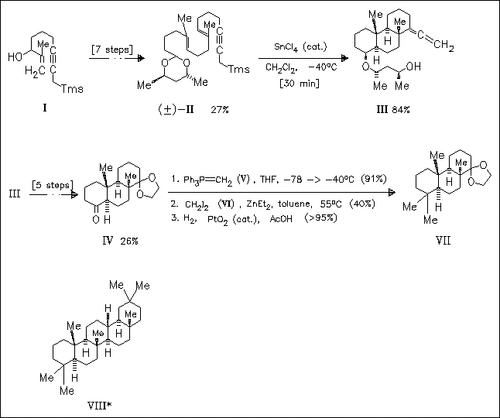
A model (IV) of the ABC rings of the oleananes is prepared using a very selective cyclization of the acetal (II) as key step. The steric hindrance by the ketone function at C-4 in (IV) allows a direct geminal dimethylation at this position. The general strategy is assumed to be transferable to the preparation of 18α(H)-oleanane (VIII) and analogues.