ChemInform Abstract: Chiral Ligands Containing Heteroatoms: Part 13. Optically Active 4-(2′- Pyridyl)-1,3-oxazolidines: An Improved Synthesis of 2-(2′-Pyridyl)-2- aminoalcohols.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
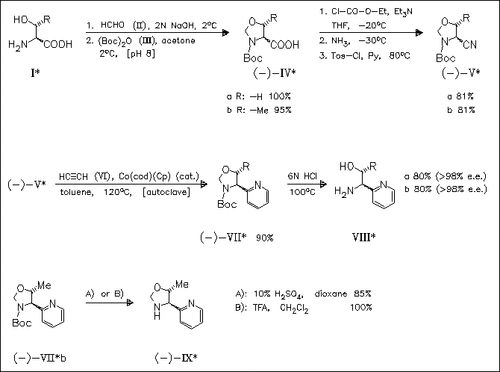
An efficient large-scale synthesis of enantiopure pyridylaminoalcohols (VIII) starts from natural amino acids (I). The key step in the short- step sequence is a Co(0)-catalyzed cocyclotrimerization of oxazolidinecarbonitrile (V) with acetylene to provide the N-protected precursors (VII) of the target compounds. Some effective methods for the selective removal of the N-protecting group with maintenance of the oxazolidine ring to give an interesting class of ligands (cf. (IX)) are also reported.