ChemInform Abstract: Claisen and Fries Rearrangement Studies of Some Naphthalene Derivatives.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
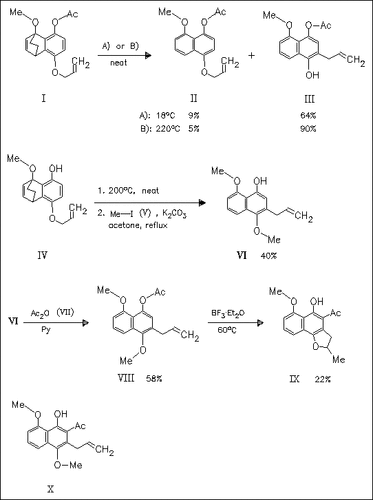
Comparative Claisen rearrangement studies are performed in order to get naphthalene-based precursors of biologically active compounds. Claisen rearrangement of the acetate (I) leads to the desired naphthalene (III) in good yields, while the corresponding 4-hydroxy derivative (IV) gives after chemoselective methylation naphthalene (VI) . Additional acylation and following Lewis acid metiated Fries rearrangement yields the furan (IX) instead of the expected naphthalene (X).