ChemInform Abstract: (2,3)-Wittig Rearrangements of Lithioalkyl Allyl Ethers Exhibit Different cis,trans-Selectivities than (2,3) Shifts in Their Lithiomethyl Analogues.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
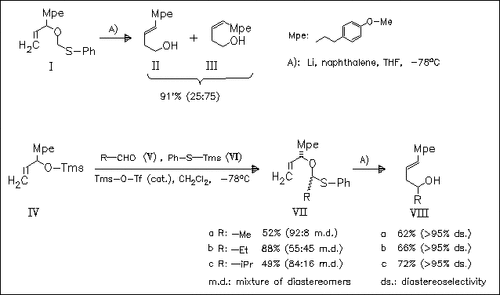
Reductive lithiation of readily achieved O,S-acetals with lithium naphthalenide initiates a (2,3)-Wittig rearrangement whose stereoselectivity depends on the structure of the starting acetal. Whilst alkyl allyl ethers (VII) give almost pure trans-configurated products, the lower homologue (I) shows a weak cis-preference. A plausible mechanistic explanation based on combined effects of the arylethyl (Mpe) and alkyl substituents (R) is given.