ChemInform Abstract: Stereoselective Syntheses of α-D- and β-D-Ribofuranosides Catalyzed by the Combined Use of Silver Salts and Their Partners.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
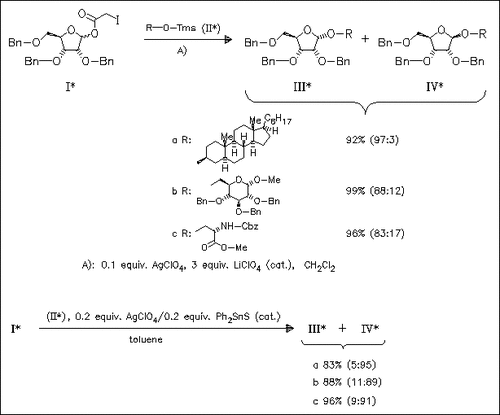
α-D-Ribofuranosides of type (III) are stereoselectively synthesized in high yields from the D-ribofuranose derivative (I) and trimethylsilylated nucleophiles such as (II) by use of AgClO4 in the coexistence of LiClO4. β-D-Ribofuranosides of type (IV) are prepared predominantly in high yields by the reaction of (I) with trimethylsilylated nucleophiles or of 2,3,5-tri-O-benzyl-D- ribofuranose with free alcohols by using Ph2SnS/Ag salt or Lawesson reagent/Ag salt combined catalyst system.