ChemInform Abstract: Synthesis and Reactions of 7-Oxonorbornane-2,3-dicarboximides.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
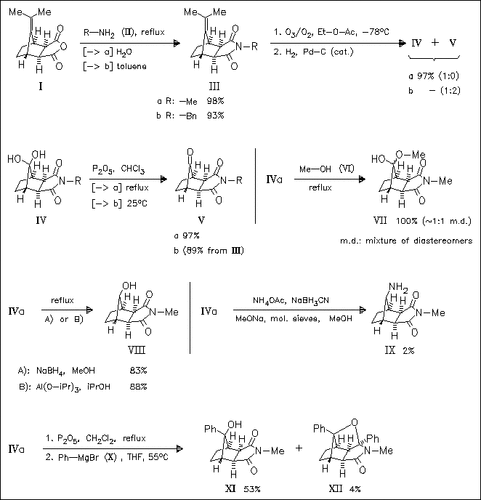
The synthesis of several 7-oxonorbornane-2,3-dicarboximides (cf. (V)), useful intermediates for the synthesis of analgetic compounds containing the perhydro-4,7-methanoisoindole skeleton, by ozonization of readily achieved 7-isopropylidenenorbornanedicarboximides (cf. (III) ) is given. These ketones, which exist preferentially in the hydrated form (cf. (IV)), react with various nucleophiles exclusively at the less hindered anti face of the ketone functionality to furnish products having syn-configuration at the C-8 position (cf. (VIII)-(XII) ). Only in the case of methanolysis of ketones or their hydrates, nearly equimolar mixtures of stereoisomeric hemiacetals (cf. (VII)) are obtained.