ChemInform Abstract: Reduction of α,β-Unsaturated Carbonyl Compounds with Sodium Borohydride in the Diethyl Ether/Methanol/Pentafluorophenol Solvent System. Use of N,N,N′,N′-Tetramethylethylenediamine and 1-Hexene as Borane Scavengers.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
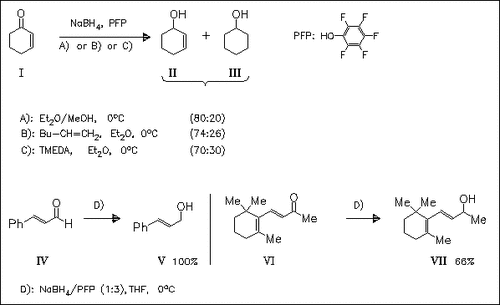
The title solvent system is used to reduce several α,β- unsaturated carbonyl compounds, e.g. (I), via 1,2-reduction to the corresponding allylic alcohols such as (II). The use of either 1- hexene or TMEDA as borane scavengers has mediocre effects on the regioselectivity. Borane formation is quenched by MeOH. Regiospecific 1,2-reduction is achieved using in situ generated sodium trispentafluorophenoxyborohydride (method D).