ChemInform Abstract: Stereoselective Protonation of Carbanions. Part 6. Enantioselective Protonation of γ-Butyrolactone Enolates.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
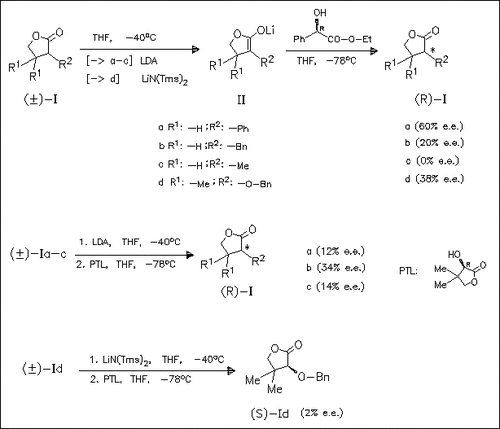
Comparisons of the enantioselectivities achieved in the protonation of lithium enolates derived from lactones (I) as well as 3- heteroanalogues (cf. foregoing papers) with chiral proton sources indicate, that definite structure-selectivity correlations can not be derived both for the enolates and the proton source.