ChemInform Abstract: (2,3)-Thia-Wittig Rearrangements of α-Lithiated Sulfides via De- Aromatized Cyclohexadiene Intermediates Proceed with Inversion of Configuration at the Carbanionic Center.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
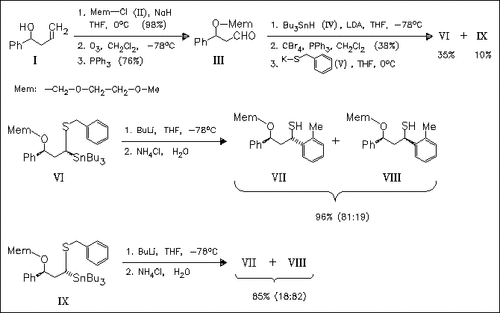
BuLi-induced Sn/Li-exchange reaction on readily achieved, diastereomeric stannylated sulfides, syn-(VI) and anti-(IX), provides the corresponding α-lithiated sulfides which undergo (2,3)-thia- Wittig rearrangement via dearomatized cyclohexadiene intermediates. Aqueous work-up of product mixtures derived from (VI) or (IX) gives the ortho-functionalized toluene derivatives (VII) and (VIII) in exactly opposite selectivity. The Wittig rearrangements presumably proceed with total inversion of configuration at the carbanion center. The incomplete stereoselectivity of the overall process is explained by an epimerization, that is 3.3 times slower than the rearrangement, of the anti/syn-α-lithio sulfides.