ChemInform Abstract: Diastereoselectivities Associated with the 1,2-Addition of Chiral ( Racemic) Cyclopentenyl Organometallics to Bicyclo(2.2.2)octenones.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
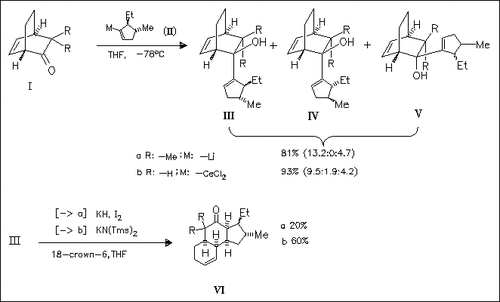
The levels of diastereoselection attainable during condensation of the bicyclooctenones (I) with organometallics, e.g. (II), derived from vinylbromides are determined. The 1,2-additions involving (Ia) exhibit excellent molecular recognition (> 98:2) during syn (endo) addition to the CO group leading mainly to the endo product (IIIa). The diasteroselectivity associated with the capture of (Ib) is lower and more variable, although still respectable in several examples. In all cases, the dominant product is the alcohol of type (III) in which the C-5 substituent on the nucleophilic subunit is β-oriented. The structural assignments follow from correlations of olefinic carbon chemical shifts, X-ray crystallography in selected examples, and anionic oxy-Cope rearrangement to generate polycyclic ketones (e.g. ( III) → (VI)).