ChemInform Abstract: New Asymmetric Synthesis of (-)-Esermethole.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
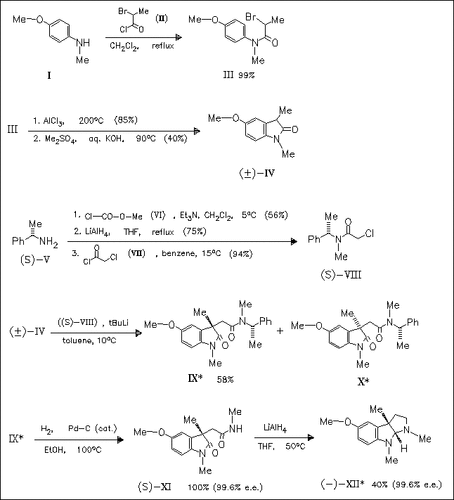
(-)-Esermethole (-)-(XII) and (+)-esermethole are synthesized with a good degree of stereoselectivity (up to 63% d.e.) by use of the (S)- phenylethylamine (S)-(VIII) and its (R)-enantiomer as chiral alkylating agents, respectively. The use of (-)-menthyl chloroacetate as chiral aklylating agent under different reaction conditions (temp., solvent and base) only leads to moderate degrees of stereoselectivity ( 1-49% d.e.).