ChemInform Abstract: Synthesis of 6-Deoxy and 3,6-Dideoxy Derivatives from Unprotected Glycosides Employing the Mitsunobu Reaction.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
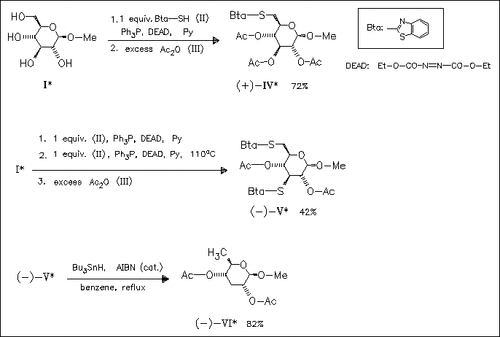
A novel and short approach for the regioselective thiofunctionalization of unprotected glycosides to furnish 6-thio (cf. (IV)) or 3,6-dithio glycosides (cf. (V)) employs the Mitsunobu reaction. Subsequent reductive desulfuration leads to the corresponding deoxy derivatives (7 examples in all). The scope of the reaction is studied with various mono- and disaccharide glycosides.