ChemInform Abstract: Lewis Acid Mediated Cyclization of ortho-Allyl Substituted Homochiral Anthraquinone Dioxolanes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
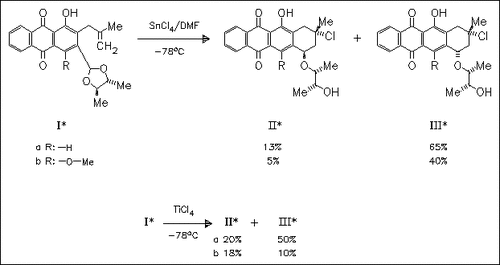
The cleavage and recyclization reaction of the 2-allyl-3-dioxolanyl- substituted anthraquinone derivatives (I) under the influence of SnCl4 or TiCl4 is investigated. Both Lewis acids favor the formation of the diastereomers (III). In this reaction, two stereocenters are generated simultaneously. Other by-products are described in the original paper.