ChemInform Abstract: Aziridines. Part 61. Ring Opening of 2,2-Dimethyl-1-sulfonylaziridines by Anilines: Regioselectivity and Revised Structure of Main Products.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
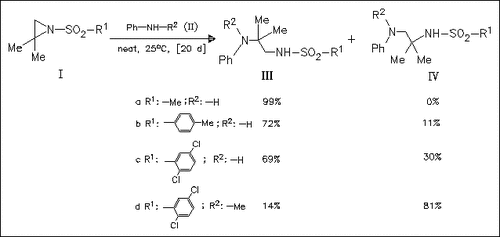
The title reaction leads to the isomeric products (III) and (IV). Using aniline, the isomers (IIIa)-(IIIc) are obtained as main products. The isomer (IV) formed by attack on position 3 of (I) usually arises in a small or negligible amount which seems to decrease with decreasing basicity of the aniline compound used and to increase with increasing steric demand of the aniline compound. On the basis of the results the mechanism of the reaction is discussed.