ChemInform Abstract: Furanoterpene Synthesis via Intramolecular Nitrile Oxide Cycloaddition Reaction: A Total Synthesis of (+)-Menthofuran.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
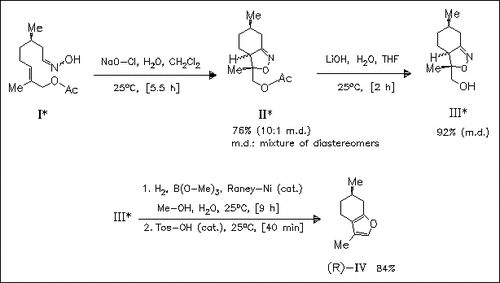
The nitrile oxide derived from the chiral oxime (R)-(I), prepared from (+)-citronellal in seven steps, undergoes an intramolecular 1,3- dipolar cycloaddition reaction to form a mixture of two diastereomeric isoxazolines (II). These are subjected to alkaline hydrolysis, catalytic hydrogenation, and acidic treatment to give (+)-menthofuran ( R)-(IV).