ChemInform Abstract: Reactions of Ethyl Phosphites with β-Nitrostyrenes. The Role of Nitrosoalkenes as Intermediates.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
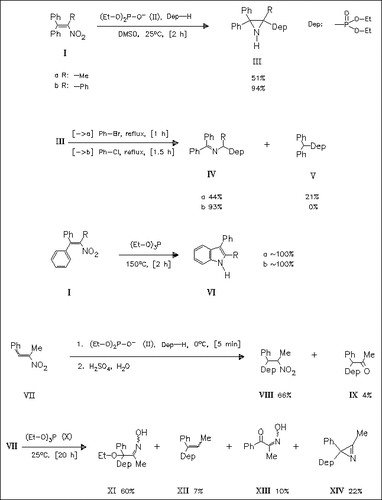
The 1-nitro-2,3-diphenylethenes (I) react with a mixture of the diethyl phosphite and the corresponding anion (II) to form the aziridinylphosphonates (III) which are thermolyzed, producing the imines (IV). In contrast, reaction of (I) with triethyl phosphite gives the indoles (VI). Furthermore, nitrostyrene derivatives such as ( VII), react with (EtO)2PO- (II)/(EtO)2P(O)H to produce the Michael type adduct (VIII). With triethyl phosphite (X), a complex reaction mixture is obtained. Some further examples are given in the original paper.