ChemInform Abstract: Quinolizidines. Part 30. A Ready Access to the Dibenzo(a,f) quinolizidine Ring System from 1,2,3,4-Tetrahydroquinoline.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
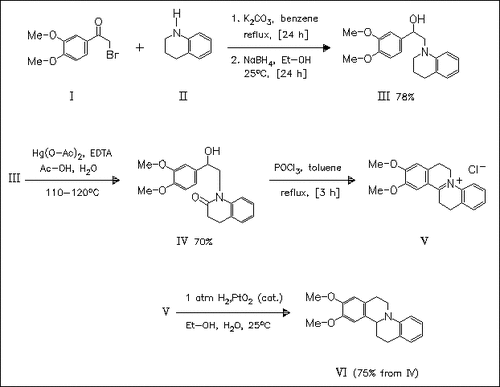
Coupling of the bromoacetophenone (I) with the tetrahydroquinoline (II) forms the corresponding amino ketone which is reduced with sodium borohydride to give the amino alcohol (III). This is oxidized with mercuric acetate to produce the lactam (IV). Reaction of (IV) with phosphoryl chloride followed by catalytical hydrogenation of the iminium chloride (V) thus obtained leads to the formation of the 9,10- dimethoxytetrahydrodibenzo(a,f)quinolizine (VI).