ChemInform Abstract: Chlorins Designed for Photodynamic Tumor Therapy and as Model Systems for Photosynthesis.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
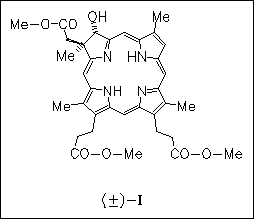
The chlorin (I) functionalized in 3 position is prepared in a few steps starting from a chlorin with geminal dialkyl substitution in the saturated pyrrole ring and a double bond in 3 position, which is easily obtained from the red blood pigment heme. The hydroxy group in ( I) is used to link a hydroquinone and an estrogen unit by means of a ( CH2)3 spacer to provide models for photosynthetic pigments as well as fluorescence markers and sensitizers for medical applications.