ChemInform Abstract: Diastereo- and Enantioselective Synthesis of trans-3-Phenyl- and trans- 3-Styryl-2-azetidinones via Zinc Ester Enolates and Imines.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
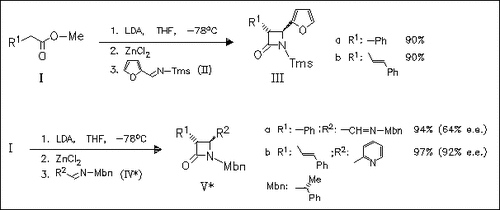
Zn enolates of phenyl- and trans-styryl-acetic acid methyl ester (I) react with excellent diastereoselectivity and good enantioselectivity ( 64 to 91.5% e.e.) with imines such as (II) and (V) to give the title compounds, e.g. (III) and (V). The corresponding Li enolates react with much lower selectivity and yields.