ChemInform Abstract: Highly Functionalized Vinylcyclopropane Derivatives by Regioselective and Stereoselective Reactions of Fischer Carbene Complexes with 1,4- Disubstituted Electron-Deficient 1,3-Dienes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
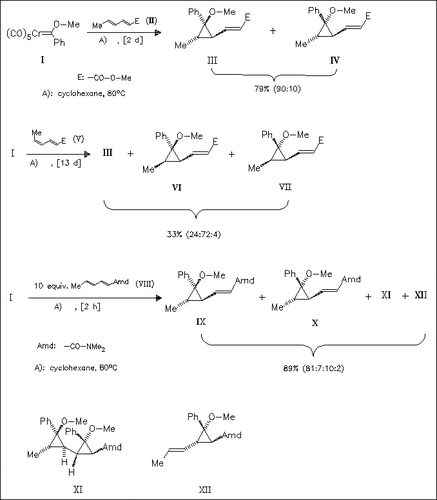
The thermal reaction of the complex (I) with 1,3-dienes such as (II) affords functionalized vinylcyclopropanes in high yield. The use of related unsaturated esters or nitriles gives similar results. There is no evidence for the formation of (4 + 1) cycloadducts and regioisomers of type (XII). The periselectivity and regioselectivity in these carbene-transfer reactions are generally very high and the diastereomer (III) with the methoxy group in cis position to the olefinic moiety is largely favored. Double adducts of type (XI) are only formed as minor side-products in some of these reactions, but when employing the amide (VIII) as the diene, they are easily using (I) in excess, in this case a formation of (IX) and (X) is not observed. From a synthetic point of view, the regioselective (2 + 1) cycloadditions described give an easy access to vinylcyclopropanes, which can serve as starting materials for further transformations.