ChemInform Abstract: Reaction of μ-Oxobis((trifluoromethanesulfonato)(phenyl)iodine(III)) with Group 14 Propargyl Derivatives and a Propargyl Ether.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
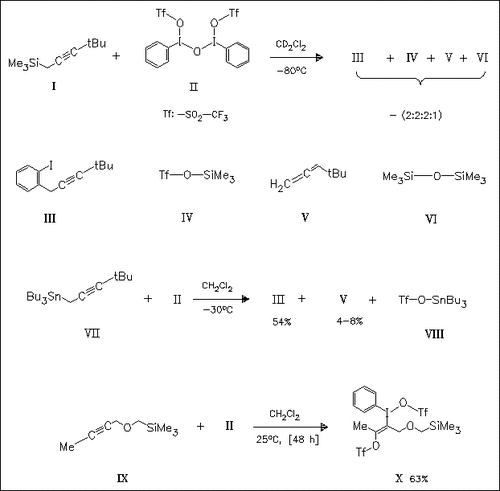
Reaction of 2 equiv. of 4,4-dimethyl-1-(trimethylsilyl)-2-pentyne (I) with the reagent (II) mentioned in the title results in the formation of 4,4-dimethyl-1-(2-iodophenyl)-2-pentyne (III), trimethylsilyl triflate (IV), tert-butylallene (V), and hexamethyldisiloxane (VI). In contrast, the addition of 2 equiv. of the 1-tributylstannyl- substituted educt (VII) to (II) gives (III), tributylstannyl triflate ( VIII) and only major amounts of the allene (V). A mechanism that explains the differences is proposed. Reaction of the ether (IX) with ( II) gives a single product (X).