ChemInform Abstract: Conformation-Dependent Cyclizations of 1,3,3,5-Tetraphenylpentane-1,5- dione.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
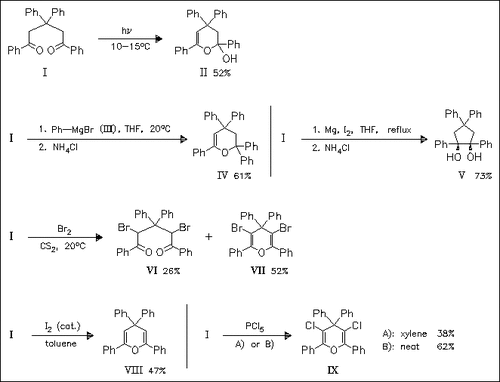
The photochemically obtained hemiacetal (II) is thermally unstable. On heating, it is transformed back to the starting dione (I). The molecular and crystal structure (space group P1, Z = 2) of the dione ( I) is investigated with respect to its cyclization reactions. Its reaction with Grignard compound (III) or halogenation with Br2 or PCl5 affords the pyran derivatives (IV), (VII), or (IX) in good yields in contrast to earlier publications, where only formation of open-chain compounds is described. On reaction with I2 the cyclic product (VIII) is obtained, whereby steric hindrance suppresses halogenation. The reduction of (I) occurs with a high degree of stereoselectivity, as shown by the formation of diol (V).