ChemInform Abstract: Diastereoselective Three-Center Michael Addition of β-Ketoesters to Prostereogenic α,β-Unsaturated Carbonyl Compounds Catalyzed by K2CO3 or Cs2CO3.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
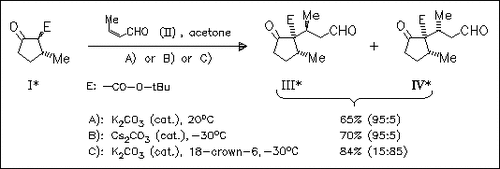
Base-catalyzed Michael additions of β-ketoesters (I) to carbonyl compounds (II) take a highly diastereoselective course giving the adducts (III), preponderantly (7 examples), thus offering a synthetically attractive approach to the Prelog-Djerassi lactone and related compounds. Complexation of the metal ions by crown ethers or other cryptands results in a reversal of diastereoselectivity affording predominantly adducts (IV) (5 examples). Possible mechanistic pathways via cyclic transition states are discussed.