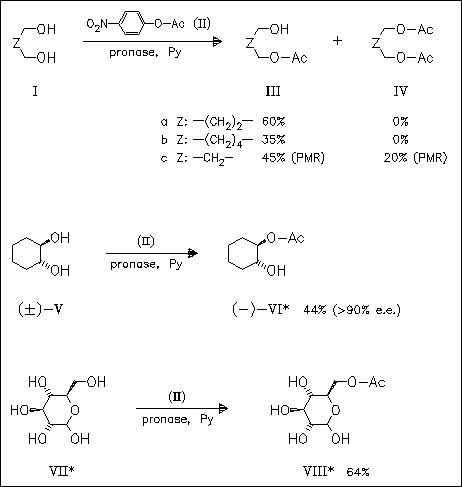ChemInform Abstract: Regio- and Stereoselective Transacylation of Polyhydric Alcohols Using Pronase in Organic Solvents.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
In the presence of pronase (mixture of proteases from Streptomyces griseus), aliphatic diols undergo acylation to afford monoacetates with exception of propane-1,3-diol, which yields the mono- and diacetate. Starting from cyclohexanediols optically active monoacetates are obtained. Glucose and isomeric hexoses are regioselectively converted into 6-O-acetates, while 6-deoxy sugars provide mixtures of monoacetates.