ChemInform Abstract: Highly Stereodivergent Approach to Both Enantiomers of 4-Substituted . beta.-Lactams via Diastereofacially Controlled Addition of Enolates of tert-Butyl Acetate to a Chiral Imine.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
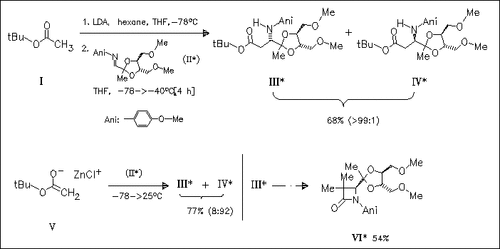
Treatment of tert-butyl acetate (I) with LDA generated in situ results in the formation of the lithium enolate which reacts with the chiral imine (II) to give the (3S)-β-amino acid ester (III) with high diastereoselectivity. On the other hand, the chlorozinc enolate (V), obtained via transmetalation of the corresponding potassium enolate of tert-butyl acetate with zinc chloride, undergoes reaction with (II) to form the (3R)-isomer (IV) as main product. These chiral amino esters, e.g. (III), can be converted to β-lactams such as (V) by literature known standard procedures without loss of the stereochemical integrity.