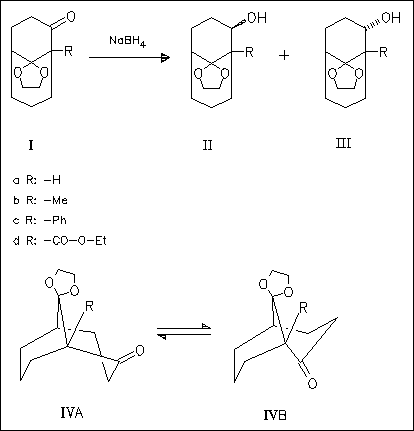
ChemInform Abstract: Conformational Effects on the Stereochemical Course of the NaBH4 Reduction of Substituted Bicyclo(3.3.1)nonan-2-ones.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
The stereochemical course of the NaBH4 reduction of (I) to exo-(II) and endo-(III) is investigated. An increase of the size of the substituent from (Ia) to (Id) effects a conformational change from ( IVa) to (IVb) (as molecular mechanics calculations show) and increases the amount of endo-(III) (according experimental results). Both, exo-( II) and endo-(III) exist in cis conformation (IVA).