ChemInform Abstract: Zirconium-Mediated Diastereoselective Coupling Reactions of Chiral Aldimine: Remarkable Temperature-Dependence of Chiral Induction.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
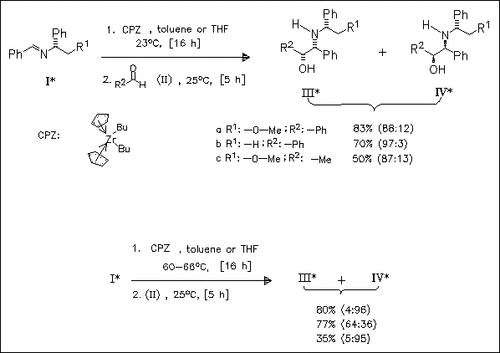
The chiral aldimines (I) react with dibutylzirconocene to form a zircconaaziridine which is further coupled with the aldehydes (II) to yield the threo-amino alcohols (III) and (IV) as the main products. The product ratio of (III) to (IV) remarkably depends on the temperature applied. When the formation of the zirconaaziridine and the subsequent coupling take place at room temperature, the isomer ( III) is obtained predominantly, whereas at 60°C the reversed selectivity is observed.