ChemInform Abstract: A Highly Diastereofacial Intramolecular Diels-Alder Reaction of α ,β-Unsaturated Carbene Complexes to Decalins.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
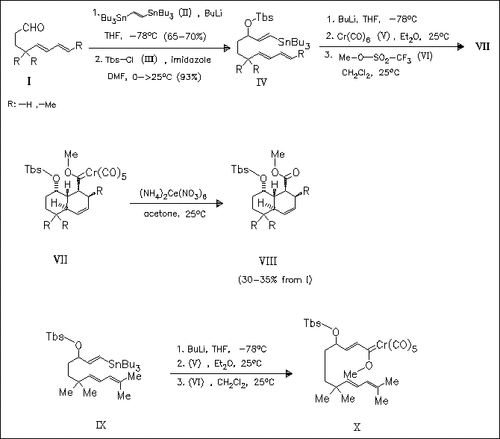
Coupling of the unsaturated aldehydes (I) with lithiated bisstannylethylene and subsequent addition of the silyl chloride (III) yield the protected trienes (IV). Further lithiation of (IV) and addition of chromium hexacarbonyl (V) lead to the formation of carbene complexes of a 1,7,9-triene type as the intermediates which undergo intramolecular Diels-Alder reactions to produce the trans-fused decalins (VII). Molybdenum and tungsten hexacarbonyls are also applied successfully in this reaction. Subsequent oxidation of (VII) with ammonium hexanitrocerate(IV) yields the esters (VIII). In the case of the tetramethyl-substituted triene (IX), the primary carbene complex ( X) is stable and cannot be converted to the corresponding decalin derivative.