ChemInform Abstract: Extremely Powerful Chiral Auxiliaries: Enantiomeric (4 + 2) Cycloadducts of 2-Oxazolone and 9,10-Dimethylanthracene.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
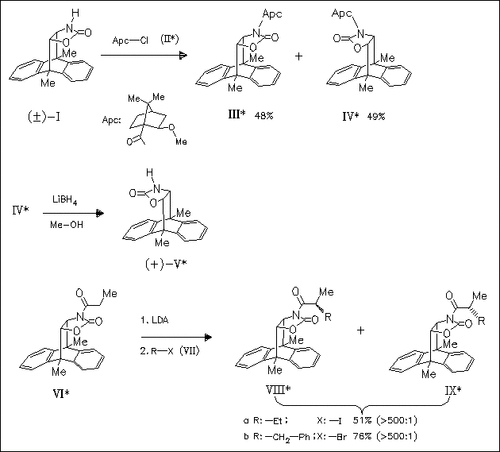
The racemic cycloadduct (I) of the uncatalyzed addition reaction of 2- oxazolone and 9,10-dimethylanthracene is coupled with (1S,2S)-2- methoxy-1-apocamphanecarboxylic chloride (II) to give a diastereomeric mixture of (III) and (IV). Further chromatographic separation on silica gel yields the pure enantiomers, e.g. (+)-(V), after deacylation by LiBH4/Me-OH. The synthetic utility of these chiral auxiliaries is demonstrated by the alkylation of the corresponding N- propionyl derivative (VI) which yields almost exclusivity the isomers ( VIII) upon lithiation and subsequent quenching with the alkyl halides ( VII).