ChemInform Abstract: Regiospecific and Highly Stereoselective Electrophilic Addition to Furanoid Glycals: Synthesis of Phosphonate Nucleotide Analogues with Potent Activity Against HIV.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
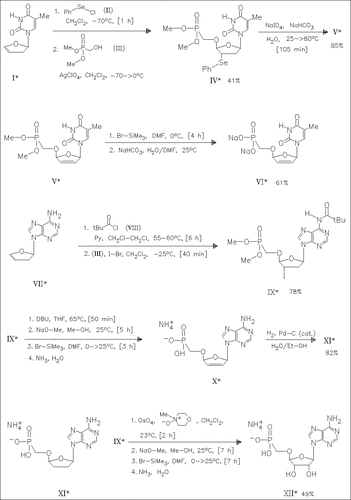
Addition of phenylselenenyl chloride (II) to the glycal derivative (I) followed by reaction with the hydroxymethylphosphonate (III) forms the phenylseleno derivative (IV). Oxidative deselenylation and ester cleavage yield the phosphonate nucleotide (VI). Consecutive reaction of the glycal (VII) with pivaloyl chloride (VIII), iodine bromide, and the phosphonate (III) produces the iodide (IX) which is converted to the phosphonate nucleotides (XI) and (XII) as outlined in the reaction scheme. The phosphonates (VI) and (X) show potent activity against retroviruses in biological tests.