ChemInform Abstract: Synthetic Approaches to 3′-Azido-3′-deoxythymidine and Other Modified Nucleosides.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
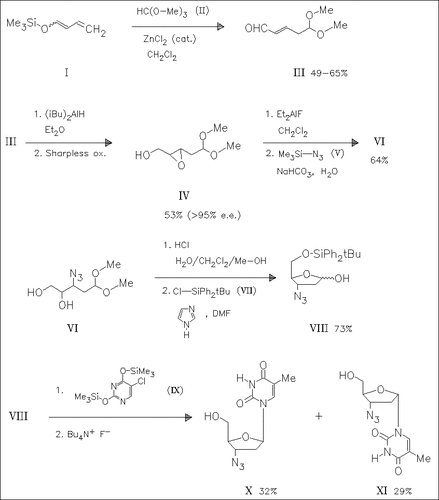
Reaction of the siloxybutadiene (I) with trimethyl orthoformate (II) forms the aldehyde acetal (III) which is converted diastereoselectively to the epoxide (IV). Treatment with trimethylsilyl azide (V) produces the azido diol (VI) which is cyclized and silylated to yield the azido sugar (VIII). Coupling with bis(trimethylsilyl)thymine (IX) and desilylation produces β-AZT ( X), together with the α-anomer (XI).