ChemInform Abstract: Nucleosides and Nucleotides. Part 97. Synthesis of New Broad Spectrum Antineoplastic Nucleosides, 2′-Deoxy-2′-methylidenecytidine (DMDC) and Its Derivatives.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
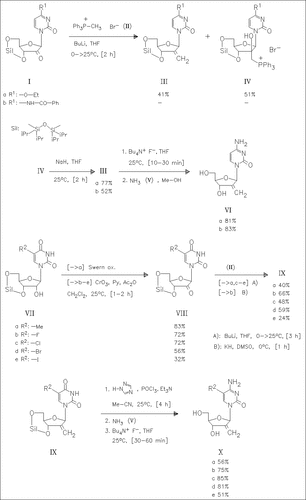
Wittig reaction of the keto nucleosides (I) with the phosphonium bromide (II) forms the methylene derivatives (III), together with the phosphonium salts (IV). The latter are converted into (III) by treatment with sodium hydride. Desilylation of (III) followed by reaction with ammonia (V) produces DMDC (VI) mentioned in the title. A series of analogues such as (X) is similarly prepared as outlined in the reaction scheme. Among the compounds prepared, the derivative (VI) and (Xb) show the best antileukemic activity against murine L1210 cells.