ChemInform Abstract: A Friedel-Crafts Cyclization Approach Toward Cephalotaxine.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
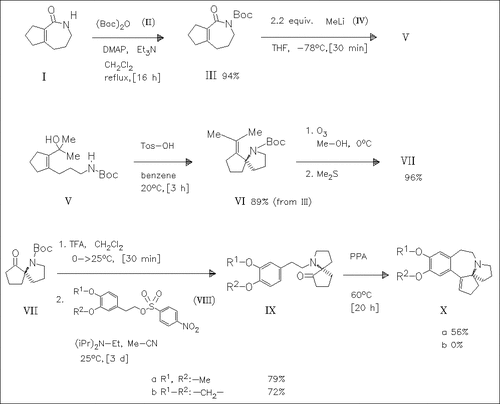
N-Protection of the azepinone (I) with the anhydride (II) followed by reaction with methyllithium (IV) yields the ring-opened carbinol (V) which is cyclized with 4-toluenesulfonic acid to produce the spiro compound (VI). Ozonolysis of (VI) forms the amino ketone (VII) which is deprotected and then coupled with the sulfonates (VIII) to give the N-alkylation products (IX). Only the derivative (IXa) is cyclized in polyphosphoric acid, producing the cephalotaxine skeleton (Xa).