ChemInform Abstract: Synthesis and Structure of Amino-Substituted Tetraphosphetanes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
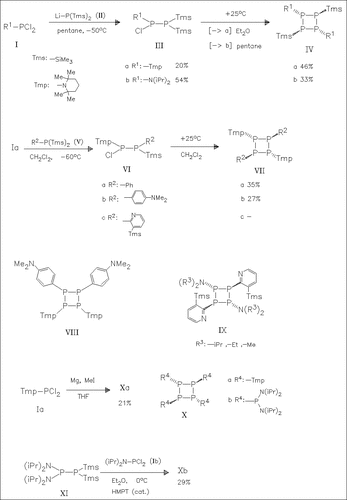
A number of aminotetraphosphetanes such as (IV), (VII)-(IX) can be obtained by elimination of chlorotrimethylsilane from initially formed vicinal chlorosilyl-functionalized biphosphines, e.g. (III) and (VI). In addition to (VIIb), its isomer (VIII) is obtained with 42% yield. The tetraphosphetane (Xa) is produced by reductive dehalogenation of ( Ia) with Mg. (2 + 2)Cyclodimerization of the labile diphosphene intermediate from (XI) and (Ib) results in the formation of the product (Xb). The structure of (IVb), (VIIa), (VIIc), (Xa), and (Xb) are determined by X-ray analysis. The torsion-angle of the four- membered ring varies depending on the size and electronegativity of the exocyclic ligands.