ChemInform Abstract: Preparation and Structural Properties of Large-Cavity Peraza Macrocycles Containing Pyridine, Phenanthroline, or Piperazine Subcyclic Units.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
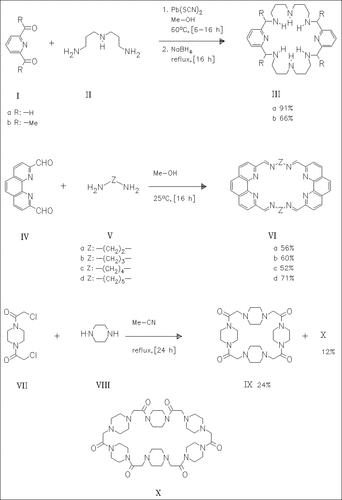
Reaction of the pyridinedicarbaldehyde (Ia) or the diacetylpyridine ( Ib) with the triamine (II) followed by reduction with sodium borohydride forms the macrocycles (III). The tetraazomethines (VI) are prepared by reaction of the phenanthrolinedicarbaldehyde (IV) with the diamines (V). Reaction of N,N′-di(chloromethylcarbonyl)piperazine (VII) with piperazine (VIII) yields the macrocycles (IX) and (X).