ChemInform Abstract: Syntheses and Thermal Behavior of 9-Substituted 9-Thia-10- azaphenanthrenes.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
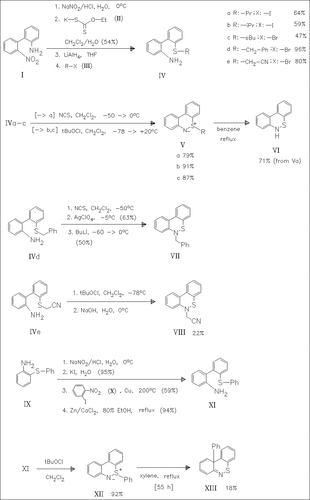
The preparation of 30 representatives of variously substituted 2- alkylthio-2′-aminobiphenyls like (IV) and (XI) is described. Some of them, e.g. (IVa)-(IVc), (XI) are converted into the compounds (V) or, resp., (XII) specified in the title and their thermal behavior is investigated. Refluxing the title compounds (V) in benzene causes . beta.-elimination to yield the dibenzo(c,e)(1,2)thiazine (VI) as the main product, whereas the title compound (XII) affords the 1,4- rearrangement product (XIII) in low yield and after prolonged reflux in xylene along with the biphenyl (XI) and starting material. It seems noteworthy that the 2-benzylthio- (IVd) and 2-cyanomethyl-substituted ( IVe) biphenyls afford the 6-substituted dibenzo(c,e)(1,2)thiazines ( VII) and, resp., (VIII) already below room temperature via 1,2- rearrangements which are rarely observed in sulfilimine chemistry.