ChemInform Abstract: Fadrozole Hydrochloride: A Potent, Selective, Nonsteroidal Inhibitor of Aromatase for the Treatment of Estrogen-Dependent Disease.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
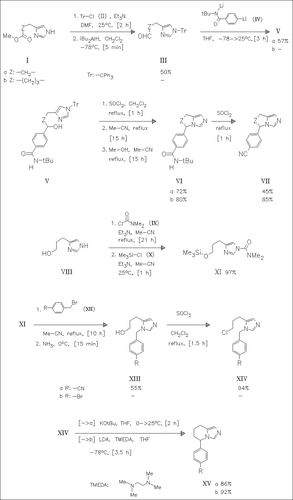
N-Protection of the imidazolealkanoates (I) followed by DIBAL reduction yields the aldehydes (III) which are coupled with the dilithium reagent (IV) to produce the alcohols (V). Subsequent cyclization of the chlorides and deprotection yield the fused imidazoles (VI) which are converted into the nitriles (VII). Bisprotection of the imidazolepropanol (VIII) followed by condensation with the benzyl bromides (XII) forms the substitution products (XIII) after deprotection. These are chlorinated and then cyclized to give the tetrahydroimidazopyridines (XV). The hydrochloride of fadrozole ( XVa) is the most potent member in the prepared series.