ChemInform Abstract: 1,2,3-Triazoles from Z-β-(Formyloxy)vinyl Azides and Triethyl Phosphite.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
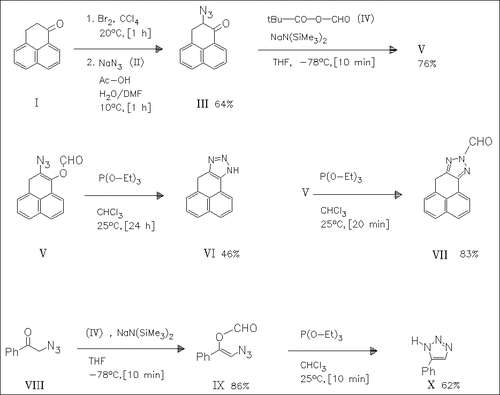
Bromination of the dihydrophenalenone (I) followed by reaction with sodium azide (II) yields the α-azido ketone (III). This compound as well as the literature-known azido ketone (VIII) are metalated by means of sodium hexamethyldisilazide and then treated with the mixed anhydride (IV) to give the (formyloxy)vinyl azides (V) and (IX) respectively which cyclize upon reaction with triethyl phosphite, forming the triazoles (VI) or (X). The N-formyltriazole (VII) is obtained upon quenching the reaction of (V) with triethyl phosphite after a short time.