ChemInform Abstract: Stereocontrolled Oxazolidinone Formation by the Addition of 4,5-Disubstituted Iminodioxolane to Oxirane via a Spiro Compound.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
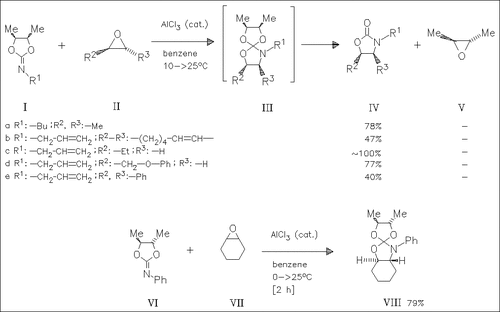
The 2-imino-1,3-dioxolanes (I) react with the oxiranes (II) in the presence of aluminum trichloride to produce the oxazolidinones (IV), together with the oxirane (V). The mechanism is discussed to proceed via the spirocyclic intermediate (III). In one case, namely by reaction of the iminodioxolane (VI) with the oxirane (VII), the spiro compound (VIII) is obtained in good yield.