ChemInform Abstract: Synthesis and Biological Evaluation of a Series of 1,1-Dichloro-2,2,3- triarylcyclopropanes as Pure Antiestrogens.
Abstract
ChemInform is a weekly Abstracting Service, delivering concise information at a glance that was extracted from about 100 leading journals. To access a ChemInform Abstract of an article which was published elsewhere, please select a “Full Text” option. The original article is trackable via the “References” option.
ChemInform Abstract
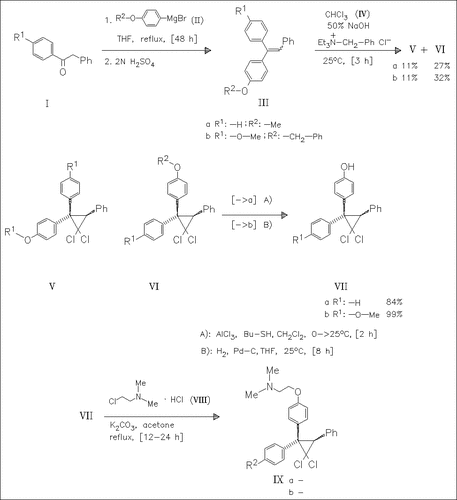
Reaction of the deoxybenzoins (I) with the 4-alkoxyphenylmagnesium bromides (II) followed by dehydration yields the triarylethylenes (III) as a mixture of E/Z-isomers. Addition of dichlorocarbene forms the dichlorocyclopropyl derivatives (V) and (VI). The latter are dealkylated and then coupled with (2-chloroethyl)dimethylamine hydrochloride (VIII) to give the corresponding O-alkylation products ( IX). Further reactions are described in the original paper. None of the tested compounds show estrogenic activity, but (IXa) and (IXb) exhibit dose-dependent antiuterotropic effects in vivo.